
在美国FDA官网上,有一份记载了近21年来检查过的GLP试验机构的清单,现从中摘出中国的清单,分享给大家。
Caveats
说 明
Inspection classifications listed in this report reflect the compliance status when the report was generated and may not represent the final Agency determination.The District Decision/Inspection Classification may be subject to change during Agency review.To maintain current knowledge of a firm's compliance status, it is important to recheck this list for updates.
本报告中列出的检查分类反映了报告产生时的遵守情况,可能不代表机构的最终决定。
地区决定/检查分类可能会在机构审查期间改变。
为了保持对公司合规性状态的最新了解,重要的是要重新检查该列表的更新情况。
The information provided should not be used as a source to compile official counts. Requests for official counts of inspections should be directed to the FDA's Division of Freedom of Information.
所提供的资料不应用作编制官方统计的来源。
官方清点检查次数的请求应直接提交给FDA的信息自由司。
The value “ORA” under the District column is not an official FDA District but may denote an inspection conducted in a foreign country or an inspection conducted by the Center.
区域栏下的值“ORA”不是正式的FDA区域,但可能表示在国外进行的检查或由本中心进行的检查。
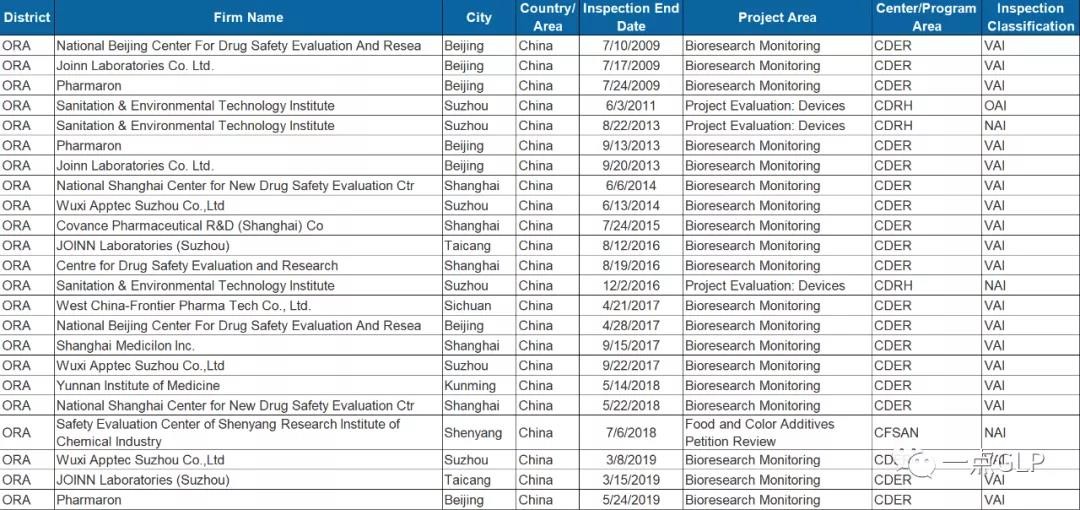
(点击图片查看大图)
来源于:一点GLP
延伸阅读
